Before digging deeper into this article, please be sure check out our article on CBD for Dogs: The Basics to learn more about CBD and THC, and the health benefits of giving CBD to your dogs.
Is CBD legal for pets?
The short answer is, well, yes and no.
The legality surrounding CBD gets a bit confusing, so we’ve also summarized all legal terms and laws in the table at the end of this article.
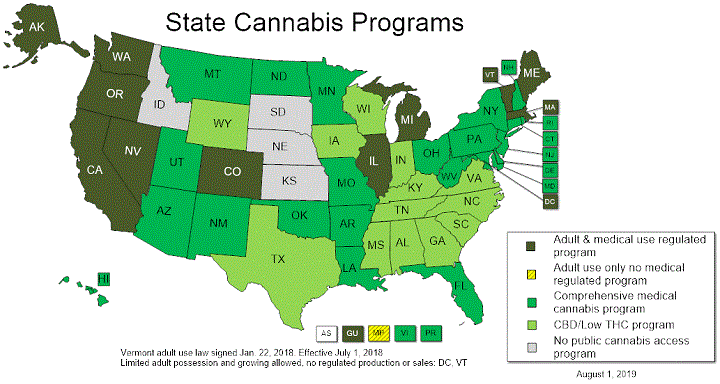
For the current legal status of cannabis in your state, check out the National Conference of State Legislatures (NCSL) website and see their map below:
Image source: NCSL1
A Brief History of CBD Legality in the US
The story of the legality of CBD is ongoing, but here’s a brief history:
1937 - Marihuana Tax Act
Placed a tax on the sale of all cannabis products. This further increased restrictions, and essentially abolished its use in veterinary medicine.2
1970 - Controlled Substance Act
Under this law, all cannabis products, including hemp, were deemed a Schedule 1 drug and illegal.3 Despite hemp not containing THC (what makes marijuana a drug), its use was banned under this Act.
2014 - “Industrial Hemp” - Agricultural Act of 2014
The groundwork for the release of hemp as a controlled substance began when Congress defined hemp in the 2014 “Farm Bill” as any part of the Cannabis sativa plant containing below 0.3% THC.4 This allowed for the use of industrial hemp for research purposes in states that permitted farming.5
2015 - FDA tests pet CBD products
The FDA found that a large majority of pet products contain CBD in amounts lower than what is listed on the label, or no CBD at all. They also found many products to be contaminated with THC.2
2018 - Agricultural Improvement Act of 2018
The 2018 “Farm Bill” released hemp and all its derivatives from the Controlled Substances Act of 1970, providing they contain less than 0.3% THC. This law federally legalized hemp-derived CBD. This Act removed the Drug Enforcement Administration’s (DEA) ability to regulate hemp as a Schedule 1 drug, but marijuana remains under their control.6 However, the Federal Food, Drug and Cosmetic Act (FD&C Act) enforced by the FDA was not impacted by this ruling, leaving hemp regulation in the hands of the FDA, allowing them to pose regulations on it as an ingredient and drug as they see fit.6
2019 - FDA rules CBD as not generally recognized as safe (GRAS)
While the control of hemp, and thus hemp-derived CBD, is out of the hands of the DEA, these agencies have the right to regulate its use as an ingredient: the FDA and the Association of American Feed Control Officials (AAFCO). All food ingredients, including in pet foods and treats, must be approved by the FDA unless they are recognized as GRAS. Since CBD was ruled as not GRAS, it is not permitted to be used as a listed ingredient in pet foods. Pet food products containing CBD are currently considered adulterated food, and can be subject to enforcement.6 The AAFCO also ruled that alongside CBD, hemp is currently not an acceptable ingredient in pet food.7,8
The FDA also issued warning letters to 15 companies selling CBD products, including those selling pet treats.9 The warning letters were mainly in protest of medical claims made around the use of CBD for pets, without CBD being approved as a drug by the FDA.6
Today -
Hemp and hemp-derived CBD are federally legal to produce. However, under the FDA and AAFCO’s ruling, CBD and hemp are federally illegal as pet food ingredients. Note, however, that hemp oil is not synonymous with hemp extract (contains CBD), and does not contain CBD. It is simply an oil produced from the seeds of the plant, while hemp CBD (extract) comes from the leaves.10
While the FDA can restrict the use of CBD in food, pet food, drugs, and human supplements, they do not have control over supplements for animals. The Dietary Supplement Health Education Act (DSHEA) does not include animal products.11
So, what if you use CBD for pets in a supplement, something that’s not considered a food or drug? That’s what many companies are doing, but the legal waters are still murky. While making claims about a product violates the FD&C Act as an unapproved animal drug,12 many companies are partnering with the National Animal Supplement Council (NASC) and following their recommendations closely.8 NASC still warns there are associated risks of selling CBD products, especially when making claims about their ability to prevent, treat, or cure a disease.11
CBD oil produced from marijuana, free of THC, can also be purchased at state dispensaries which permit adult use of cannabis.13 However, marijuana was not released from the CSA under the 2018 Farm Bill, so while CBD can be extracted from either cannabis plant, hemp-derived CBD vs marijuana-derived CBD, even though both are free of THC, have different legal standings.3
All this said, these are federal regulations. Many states still have laws on the books which make hemp illegal within the state. Although prosecution for having or recommending hemp products in those states is unlikely, it gives some people pause. This has been the opposite issue for medical marijuana, when states legalize and the federal government still bans it. There is a lot of tension between state and federal laws in the cannabis industry!
Laws and Legal Terms surrounding CBD
| Agricultural Improvement Act of 2018 | “2018 Farm Bill” - Legalized hemp (+ hemp-derived products) containing < 0.3% THC, federally, which was previously a Schedule 1 drug under the Controlled Substances Act of 1970. Legalized hemp-derived CBD. |
|---|---|
| Drug Enforcement Administration (DEA) | Federal law enforcement agency, that regulates drug distribution in the US. Lost the ability to regulate hemp derived CBD, under 2018 Farm Bill, which is now regulated by the FDA. |
| Controlled Substances Act (CSA) | 1970 Act that made all cannabis plants federally illegal, including hemp and marijuana, labeling them as Schedule 1 controlled substances. 2018 Farm Bill released hemp. |
| Schedule 1 drug | Illegal drugs considered to have high abuse potential and safety issues. Still includes marijuana. |
| Federal Food Drug & Cosmetic Act (FD&C Act) | Set of laws passed by Congress in 1938 that provided the FDA with the authority to regulate food, drugs, medical devices, and cosmetics. Was not impacted by the 2018 Farm Bill, keeping the FDAs power intact to regulate hemp and its derivatives, including hemp-derived CBD. |
| Food and Drug Administration (FDA) | Federal agency of the US Department of Health and Human Services. Evaluates and approves ingredients in food, including pet food. Considers CBD an unapproved food ingredient that is not GRAS or accepted by the AAFCO. Further prohibits the marketing of medical claims for CBD. |
| Generally Recognized as Safe (GRAS) | All food ingredients must be approved by the FDA unless they have been recognized as GRAS. FDA ruled CBD is not considered GRAS. |
| Association of American Feed Control Officials (AAFCO) | Voluntary organization, with no official means of enforcement, but considered vital by FDA and regulations are adopted by most states. Evaluates animal feeds and pet foods, and provides ingredient, nutrition, and label requirements. Considers CBD an unacceptable ingredient because it is not GRAS. |
| National Animal Supplement Council (NASC) | Nonprofit industry group working to protect the health of companion animals in the US, with strict compliance and quality standards for animal supplements. Partners with some companies looking to market CBD products, providing strict guidelines. |
References:
- Karmen Hanson AG. State Medical Marijuana Laws. 16 Oct 2019 [cited 11 Dec 2019]. Available: http://www.ncsl.org/research/health/state-medical-marijuana-laws.aspx
- Brutlag A, Hommerding H. Toxicology of Marijuana, Synthetic Cannabinoids, and Cannabidiol in Dogs and Cats. Vet Clin North Am Small Anim Pract. 2018;48: 1087–1102.
- Cadena A. Hemp vs Marijuana: The Difference Explained. In: Medium [Internet]. CBD Origin; 10 Sep 2018 [cited 4 Jan 2020]. Available: https://medium.com/cbd-origin/hemp-vs-marijuana-the-difference-explained-a837c51aa8f7
- Rumple S. High Time for cannabis research. Trends Magazine, American Animal Hospital Association. 2018; 29–34.
- Guiden M. Preliminary data from CBD clinical trials “promising.” In: News from the College of Veterinary Medicine and Biomedical Sciences [Internet]. 20 Jul 2018 [cited 19 Dec 2019]. Available: https://cvmbs.source.colostate.edu/preliminary-data-from-cbd-clinical-trials-promising/
- Dzanis DA, DVM, DACVN. CBD in pet foods: Has anything changed with legal status? In: PetfoodIndustry.com [Internet]. 9 Dec 2019 [cited 19 Dec 2019]. Available: https://www.petfoodindustry.com/articles/8751-cbd-in-pet-foods-has-anything-changed-with-legal-status?v=preview
- Annis M, Lyon E. Hemp, CBD ingredients in pet foods proves risky business. [cited 11 Jan 2020]. Available: https://www.petfoodprocessing.net/articles/13182-hemp-cbd-ingredients-in-pet-foods-proves-risky-business?v=preview
- Beaton L. Petfood Industry - December 2019. [cited 20 Dec 2019]. Available: http://www.petfoodindustry-digital.com/201912/index.php#/18
- Wall T. CBD not Generally Recognized as Safe (GRAS) for pet foods. In: PetfoodIndustry.com [Internet]. 2 Dec 2019 [cited 30 Dec 2019]. Available: https://www.petfoodindustry.com/articles/8730-cbd-not-generally-recognized-as-safe-gras-for-pet-foods
- CBD Oil for Dogs. In: Innovet Pet Products I Best Hemp Products for Pets [Internet]. [cited 7 Jan 2020]. Available: https://www.innovetpet.com/products/purcbd
- NASC’s Perspective on Hemp & CBD. [cited 5 Jan 2020]. Available: http://www.petbusiness.com/NASCs-Perspective-on-Hemp-CBD/
- Wall T. How CBD pet product brands avoid federal warnings. In: PetfoodIndustry.com [Internet]. 6 Jan 2020 [cited 8 Jan 2020]. Available: https://www.petfoodindustry.com/articles/8793-how-cbd-pet-product-brands-avoid-federal-warnings
- Gleichmann N. Hemp vs Marijuana: Is There a Difference? In: Analytical Cannabis [Internet]. Analytical Cannabis; 2 Sep 2019 [cited 5 Jan 2020]. Available: https://www.analyticalcannabis.com/news/hemp-vs-marijuana-is-there-a-difference-311880